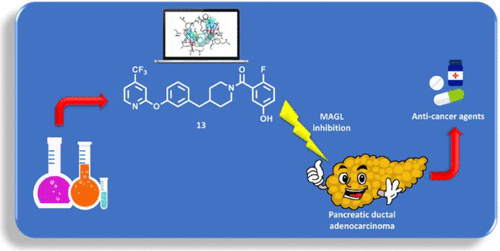
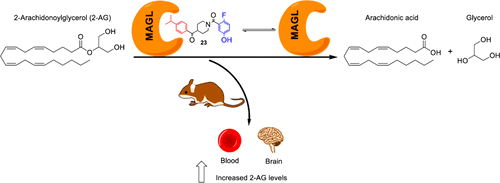
Monoacylglycerol lipase (MAGL) is a serine hydrolase that play a major role in the degradation of 2-arachidonoylglycerol, an endocannabinoid neurotransmitter implicated in several physiological processes. Recent studies have shown the possible role of MAGL inhibitors as anti-inflammatory, anti-nociceptive and anti-cancer agents.
Part of our team is dedicated to develop new potent and selective reversible MAGL inhibitors. Furthermore, we are open to the collaboration with professors and researchers at universities worldwide that want to be involved in the identification of new MAGL inhibitors.
We welcome all the possible collaborations. We can provide a) in silico screening, b) experimental screening on the MAGL target, c) cellular assays and d) we can also supply reversible MAGL inhibitors for in vivo experiments.
For requesting more information, please send an email using the Contacts form.
Publications:
198.
Reversible Monoacylglycerol Lipase Inhibitors: Discovery of a New Class of Benzylpiperidine Derivatives. Bononi G, Di Stefano M, Poli G, Ortore G, Meier P, Masetto F, Caligiuri I, Rizzolio F, Macchia M, Chicca A, Avan A, Giovannetti E, Vagaggini C, Brai A, Dreassi E, Valoti M, Minutolo F, Granchi C, Gertsch J, Tuccinardi T. J Med Chem. 2022, 65(10):7118-7140.
191.
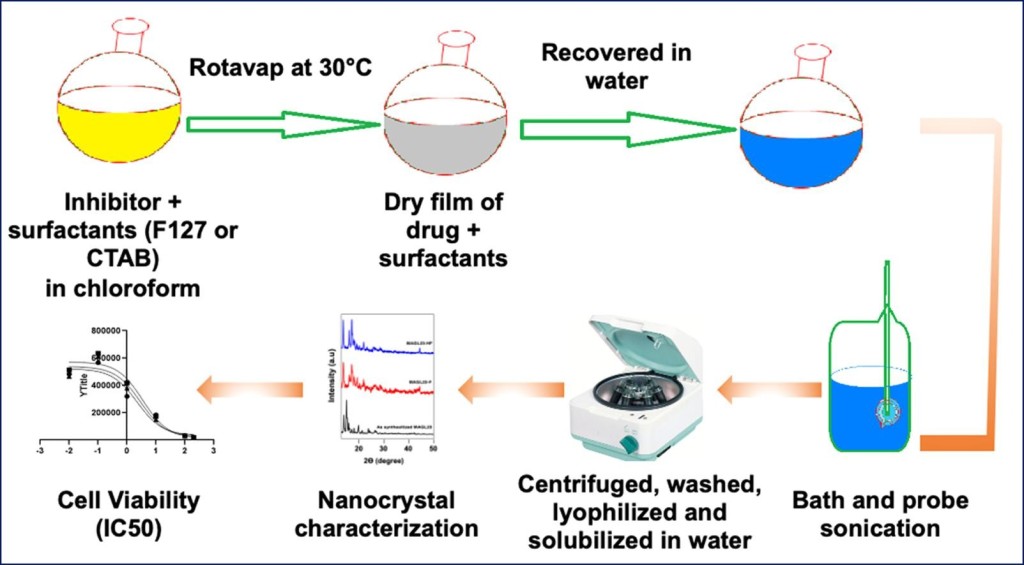
A Carrier Free Delivery System of a Monoacylglycerol Lipase Hydrophobic Inhibitor. Adeel M, Saorin G, Boccalon G, Sfriso AA, Parisi S, Moro I, Palazzolo S, Caligiuri I, Granchi C, Corona G, Cemazar M, Canzonieri V, Tuccinardi T, Rizzolio F. Int J Pharm. 2022, 613:121374.
182.
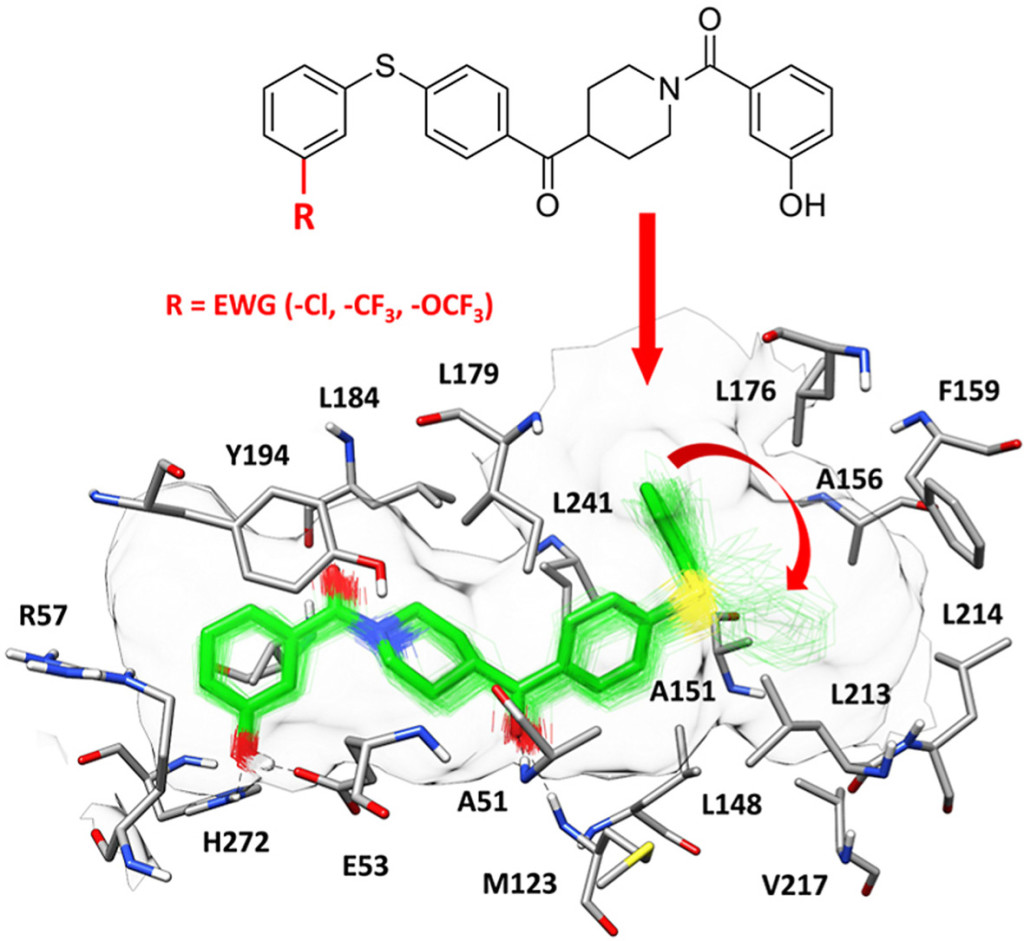
Monoacylglycerol lipase (MAGL) inhibitors based on a diphenylsulfide-benzoylpiperidine scaffold. Bononi G, Tonarini G, Poli G, Barravecchia I, Caligiuri I, Macchia M, Rizzolio F, Demontis GC, Minutolo F, Granchi C, Tuccinardi T. Eur J Med Chem. 2021, 223:113679.
175.
An updated patent review of monoacylglycerol lipase (MAGL) inhibitors (2018-present). Bononi G, Poli G, Rizzolio F, Tuccinardi T, Macchia M, Minutolo F, Granchi C. Expert Opin Ther Pat. 2021, 31(2):153-168.
172.
Design, synthesis and biological evaluation of second-generation benzoylpiperidine derivatives as reversible monoacylglycerol lipase (MAGL) inhibitors. Granchi C, Bononi G, Ferrisi R, Gori E, Mantini G, Glasmacher S, Poli G, Palazzolo S, Caligiuri I, Rizzolio F, Canzonieri V, Perin T, Gertsch J, Sodi A, Giovannetti E, Macchia M, Minutolo F, Tuccinardi T, Chicca A. Eur J Med Chem. 2021, 209:112857.
137.
Optimization of a Benzoylpiperidine Class Identifies a Highly Potent and Selective Reversible Monoacylglycerol Lipase (MAGL) Inhibitor. Granchi C, Lapillo M, Glasmacher S, Bononi G, Licari C, Poli G, El Boustani M, Caligiuri I, Rizzolio F, Gertsch J, Macchia M, Minutolo F, Tuccinardi T, Chicca A. J Med Chem. 2019, 62(4):1932-1958.
135.
Computationally driven discovery of phenyl(piperazin-1-yl)methanone derivatives as reversible monoacylglycerol lipase (MAGL) inhibitors. Poli G, Lapillo M, Jha V, Mouawad N, Caligiuri I, Macchia M, Minutolo F, Rizzolio F, Tuccinardi T, Granchi C. J Enzyme Inhib Med Chem. 2019, 34(1):589-596.
129.
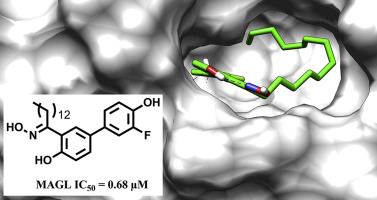
Discovery of long-chain salicylketoxime derivatives as monoacylglycerol lipase (MAGL) inhibitors. Bononi G, Granchi C, Lapillo M, Giannotti M, Nieri D, Fortunato S, Boustani ME, Caligiuri I, Poli G, Carlson KE, Kim SH, Macchia M, Martinelli A, Rizzolio F, Chicca A, Katzenellenbogen JA, Minutolo F, Tuccinardi T. Eur J Med Chem. 2018, 157:817-836.
119.
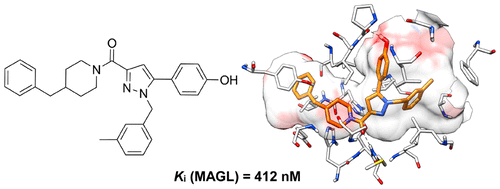
Discovery of 1,5-Diphenylpyrazole-3-Carboxamide Derivatives as Potent, Reversible, and Selective Monoacylglycerol Lipase (MAGL) Inhibitors. Aghazadeh Tabrizi M, Baraldi PG, Baraldi S, Ruggiero E, De Stefano L, Rizzolio F, Di Cesare Mannelli L, Ghelardini C, Chicca A, Lapillo M, Gertsch J, Manera C, Macchia M, Martinelli A, Granchi C, Minutolo F, Tuccinardi T. J Med Chem. 2018, 61(3):1340-1354.
116.
A patent review of Monoacylglycerol Lipase (MAGL) inhibitors (2013-2017). Granchi C, Caligiuri I, Minutolo F, Rizzolio F, Tuccinardi T. Expert Opin Ther Pat. 2017, 27(12):1341-1351.
114.
Development of terphenyl-2-methyloxazol-5(4H)-one derivatives as selective reversible MAGL inhibitors. Granchi C, Caligiuri I, Bertelli E, Poli G, Rizzolio F, Macchia M, Martinelli A, Minutolo F, Tuccinardi T. J Enzyme Inhib Med Chem. 2017, 32(1):1240-1252.
104.
Structural optimization of 4-chlorobenzoylpiperidine derivatives for the development of potent, reversible and selective monoacylglycerol lipase (MAGL) inhibitors. Granchi C, Rizzolio F, Palazzolo S, Carmignani S, Macchia M, Saccomanni G, Manera C, Martinelli A, Minutolo F, Tuccinardi T. J Med Chem. 2016, 59(22):10299-10314.
93.
4-Aryliden-2-methyloxazol-5(4H)-one as a new scaffold for selective reversible MAGL inhibitors. Granchi C, Rizzolio F, Bordoni V, Caligiuri I, Manera C, Macchia M, Minutolo F, Martinelli A, Giordano A, Tuccinardi T. J Enzyme Inhib Med Chem. 2016, 31(1):137-146.
76.
Identification and characterization of a new reversible MAGL inhibitor. Tuccinardi T, Granchi C, Rizzolio F, Caligiuri I, Battistello V, Toffoli G, Minutolo F, Macchia M, Martinelli A. Bioorg Med Chem. 2014, 22(13):3285-3291.